A-Level Chemistry (F334) (What's in a Medicine?) Note on Thin Layer Chromatography - Summary Notes, created by Carter Bartlett on 21/12/2014.
Pinned to
168
1
0
No tags specified
|
|
Created by Carter Bartlett
over 10 years ago
|
|
Rate this resource by clicking on the stars below:




 (0)
(0)
Ratings (0)
| 0 | ||
| 0 | ||
| 0 | ||
| 0 | ||
| 0 |
0 comments
There are no comments, be the first and leave one below:
Close
1813418
note
2016-02-19T07:33:21Z
1/1
TLC - Thin Layer Chromatography
All chromatography techniques consists of a stationary phase, through which an inert mobile phase travels. The mixture is added to the mobile phase and seperates as it's constituents travel at different speeds. Below is a typical TLC setup.
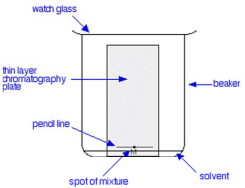
Within the above diagram, the thin layer chromatography plate (TLC plate), would be regarded as our stationary phase. And the solvent would be regarded as our mobile phase.
All TLC techniques follow a 6-step process to complete.
All TLC techniques follow a 6-step process to complete.
- Draw a pencil-line near the bottom of the TLC plate
- A small sample of the mixture is place on the line
- The TLC plate is placed in the solvent, ensuring the line is above the solvent
- Cover the beaker using a watch glass
- As the solvent nears the top of the TLC plate, remove the plate and leave it to dry
- Locate the spots on the TLC plate using Iodine, UV light or Ninhydrin

The line at the top of the TLC plate is the maximum height the solvent reached, known as the solvent front. Each of the spots on the TLC plate refers to a seperate Rf value. To calculate this Rf value, we use the following formula
Rf value = distance spot travelled / distance solvent travelled
Thin layer chromatography - summary notes
