Some common precipitates
Pinned to
94
0
0

|
Created by Peter Hoskins
about 10 years ago
|
|
Rate this resource by clicking on the stars below:




 (0)
(0)
0 comments
There are no comments, be the first and leave one below:
Close
2853513
note
2016-11-28T00:31:46Z
1/1
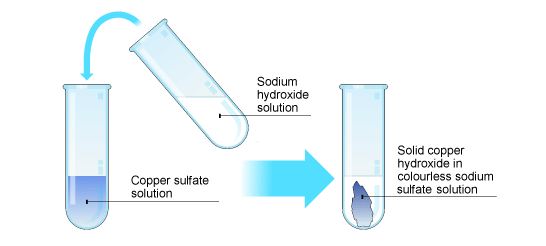
Precipitation reactions
Transition metal hydroxides are insoluble in water.
If a solution of any soluble transition metal compound is mixed with sodium hydroxide solution then there is displacement reaction. The sodium is the more reactive metal, and displaces the transition metal from its compound. The transition metal hydroxide is produced as a result. As this is insoluble in water it appears as a solid in the liquid. A solid produced in a liquid in this way is called a precipitate.