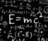Chem 2.3
Resumo de Recurso
Página 1
Exothermic reactions - heat is lost to surroundings - the enthalpy change is negativeEndothermic reactions - heat is taken in from surroundings - the enthalpy change is positive
Activation Energy is the minimum energy needed for a reaction to start
Q = mcAT ( A = delta )Energy Change = mass x specific heat capacity x temperatureEnergy Change = J Enthalpy Change kJ/mol
Breaking bonds requires energyMaking bonds releases energy
Calculating enthalpy change from bond enthalpiestotal bond enthalpy of bonds broken - total bond enthalpy of bonds formed
http://edgecastcdn.net/000031/DLStudent(online)/_TestVersions/Edexcel_Chemistry_for_AS/Resources/EDX...
Hess' Law
Collision TheoryIncreasing concentration increases the number of molecules in the same volume.Therefore there are more frequent collisions and the rate of reaction increasesIncreasing pressure is pushing gas molecules closer together(the same number of molecules occupy a smaller volume). Therefore there are more frequent collisions and the rate of reaction increases
CatalystsSpeed up the rate of reaction without being consumed by the reactionCatalysts lower activation energy therefore reduce the temperature needed for the reaction.Allow better reactions with high atom economy and less waste but would otherwise require too much energy
Boltzmann distributiony axis - Number of moleculesx axis - EnergyIncreasing temperature increases the Energy of the molecules - flattening the curve , more molecules have energy above the activation energy.Catalysts reduce the activation energy - increasing number of molecules with energy above the activation energy
Dynamic Equilibrium - when the rate of r=forward reaction = rate of backward reaction.
Catalysts have no effect on position of equilibrium
Enthalpy Changes
Rates of Equilibrium
Quer criar suas próprias Notas gratuitas com a GoConqr? Saiba mais.